
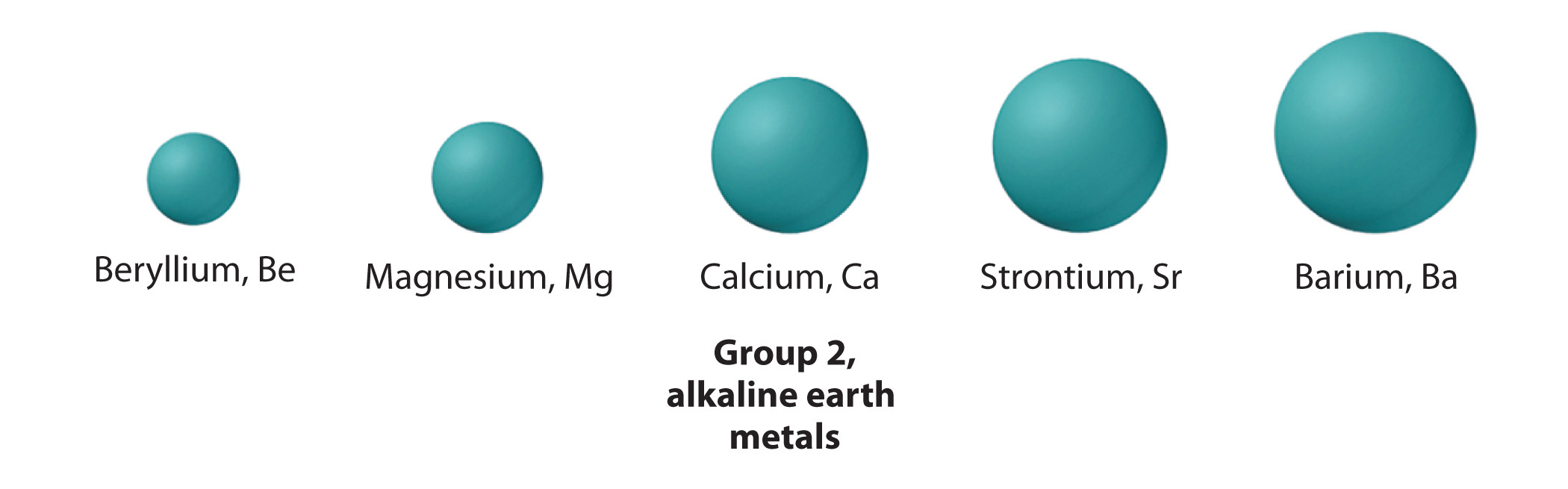
It is one the distance which is half the distance between the nuclei of two similar non-bonded isolated atoms or even two adjacent identical atoms belonging to two neighboring molecules of an element which are in the solid-state. The internuclear distance which is between two bonded atoms is called the bond length. Here letter r covalent is defined as = ½ that is the internuclear distance between two bonded atoms. The radius of atoms is more than 10,000 times the radius of its nucleus that is 1–10 pm and less than 1/1000 of the wavelength of visible light that is 400–700 nm. In most of the definitions of the radii of isolated atoms which are neutral atoms range between 30 and 300 pm that is trillionths.
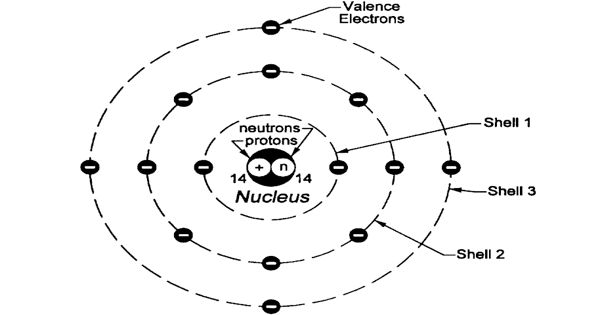
In condensed molecules and matter the cloud of electrons of the atoms usually overlap to some extent, and some of the electrons can even roam over a large region encompassing two or even more atoms. The position of the molecules can be described as probability which has distributions that gradually taper off as one that moves away from the nucleus. It is said that the electrons do not have definite orbits or sharply defined ranges. The radius value can even depend on the atom's state. And its value can also be obtained by the experimental measurements, or computed from models of theory. There are three widely used definitions which are used for atomic radius:they are the ionic radius, Van der Waals radius, and covalent radius.Īccording to the definition these terms may be applied only to isolated atoms in condensed matter as well covalently which have bonding in molecules or even within ionized and excited states as well. The meaning of it is said as the typical distance which is from the center of the nucleus till the boundary of the atom which is surrounding the electrons. The radius of an atom of a chemical element is a measure of the atom's size.


 0 kommentar(er)
0 kommentar(er)
